anatomy and function of larval brain
Most of the Drosophila learning and memory studies utilize adult flies based on the Seymour Benzer’s pioneering work. Although simpler than the human brain, its complex neuropils set an obstacle to the identification of the exact neural circuitry. In contrast, Drosophila larvae have a profoundly simple brain yet exhibit a variety of intriguing behaviors. Besides, whereas the adult brain is formed through complex reorganization during metamorphosis, the larval brain is a simple extension of the embryonic axonal plan, and thus constitutes an attractive system for elucidation of the underlying neurocircuitry mechanisms involved in learning and memory. We have developed a novel olfactory associative paradigm using gustatory reinforcing cues such as sucrose or quinine (Honjo and Furukubo-Tokunaga, 2005; Honjo and Furukubo-Tokunaga, 2009). Mutant analyses have suggested the importance of cAMP signaling and potassium channel activities in larval learning as in adult fly learning. Intriguingly, effective retention of larval memory produced with the appetitive conditioning also requires the activities of Amnesiac and cAMP response element-binding (CREB) proteins. Moreover, the synaptic output of mushroom body neurons is required for retrieval but not for acquisition and retention of the larval memory including the CREB-dependent component (Honjo and Furukubo-Tokunaga, 2005).
memory circuits in the larval brain
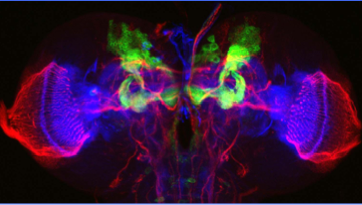
Drosophila larval brain.
Green: mushroom body as revealed with a GAL4 driver (201Y)
Red: major axonal tracts labeled by anti-Fas II antibody
Blue: actin fibers stained with phalloidin
Late 3rd instar stage.
Kurusu, M. et al., 2002
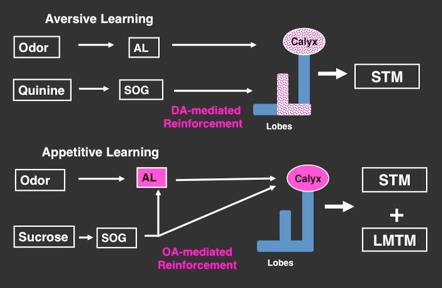
Distinctive pathways of aversive and appetitive memory formation.
Unlike the complexity found in the adult brain, the organization of the larval olfactory system is straightforward, lacking cellular redundancy. Odor information sensed by olfactory sensory neurons is transmitted to specific sets of the antennal lobe (AL) glomeruli, and further conveyed to the calyx, the dendritic structure of the mushroom body.
Aversive pathway; the bitter sensing neurons project to SOG, the primary gustatory center, from which the aversive information is transmitted to the modulatory dopamine (DA) neurons, which project on the mushroom body lobes. Convergence of the odor and the aversive signals may be achieved via spatially separate inputs on the different parts of the mushroom body. Only short-term memory (STM) is formed with the aversive reinforcement.
Appetitive pathway; the sucrose reward stimuli sensed by sugar-responsive gustatory neurons is first transmitted to SOG and is transmitted to the modulatory octopamine (OA) neurons, which project in duplicate onto the dendritic structures of both the antennal lobe and the mushroom body. The reward information may thus be associated with the odor information both at the antennal lobe glomeruli and the mushroom body calyx, leading to the formation of STM and a larval medium-term memory (LMTM).
Honjo and Furukubo-Tokunaga, 2009
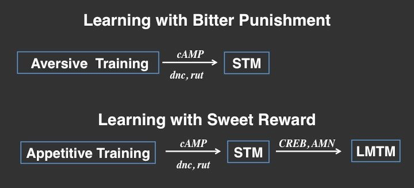
Comparative studies of memory stability in larvae revealed differential retention of olfactory memory formed with aversive and appetitive reinforcing stimuli (Honjo and Furukubo-Tokunaga, 2005; Honjo and Furukubo-Tokunaga, 2009). Thus, while appetitive memory generated with sucrose is maintained for 120 min, aversive memory induced with quinine is lost in 20 min, even with the same odor and under an identical training paradigm. Neurocircuitry analyses show that synaptic output of octopaminergic and dopaminergic neurons, which exhibit distinctive innervation patterns in the mushroom body and the antennal lobe, is differentially required for the acquisition of appetitive and aversive memory, respectively (Honjo and Furukubo-Tokunaga, 2009). These results suggest that genetically programmed memory circuitries constructed in early development provide predisposition in the efficacy of inducing longer-lived memory components in the larval brain.
FURUKUBO-TOKUNAGA LABORATORY
INSTITUTE OF BIOLOGICAL SCIENCES, UNIVERSITY OF TSUKUBA, JAPAN
Update June 2018
Induction of artificial olfactory memory in larvae
Based on these findings, we attempted whether it is possible to generate artificial memory in larvae. It has been postulated that associative memory is formed by at least two sets of external stimuli, CS and US, that are transmitted to the memory centers by distinctive conversing pathways. However, whether associative memory can be induced by the activation of only the olfactory CS and a biogenic amine-mediated US pathways remains to be elucidated. To directly examine the hypothesis, we substituted the reward signals with dTrpA1-mediated thermogenetic activation of octopaminergic neurons and the odor signals by ChR2-mediated optical activation of a specific class of olfactory neurons. We have shown that targeted activation of the olfactory receptor and the octopaminergic neurons is indeed sufficient for the formation of associative olfactory memory in the larval brain. We could also show that targeted stimulation of only a single type of olfactory receptor neurons is sufficient to induce olfactory memory that is indistinguishable from natural memory induced by the activation of multiple olfactory receptor neurons (Honda et al., 2014; Honda et al., 2016).
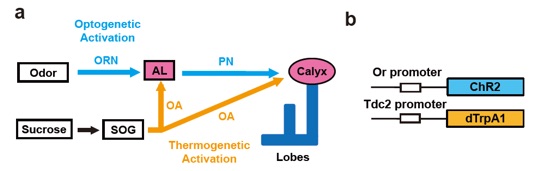
(a) Schematic diagram for artificial induction of associative olfactory memory in the fruit fly larvae. This method consists of 1) substitution of reward signals with dTrpA1-mediated thermogenetic activation of OA neurons and 2) substitution of the odor signals with ChR2-mediated optical activation of a specific class of ORNs.
(b) Structures of the Or-ChR2 and Tdc2-dTrpA1 constructs. Or-ChR2 construct; the coding sequence of ChR2 is directly placed under the transcriptional promoter sequence of an Or gene, which is expressed in a specific set of the larval ORNs. Tdc2-dTrpA1 construct; the coding sequence of dTrpA1 is fused with the transcriptional promoter sequence of the Tdc2 gene, which is expressed in the larval OA neurons.
Honda et al., 2014; Honda et al., 2016
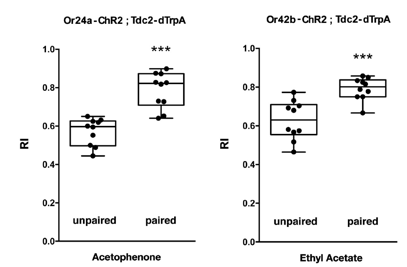
Odor specificity of artificial memory.
Olfactory responses of transgenic larvae (Or-ChR2; Tdc2-dTrpA1) with unpaired and paired conditioning. The paired but not the unpaired conditioning with blue light and heat (28˚C) caused a significant increase in the olfactory response to the specific odorant determined by the Or-ChR2 type: acetophenone for Or24a and ethyl acetate for Or42b. ***p < 0.001 by Mann-Whitney U-test between unpaired and paired group. n = 10 trials.
Honda et al., 2016
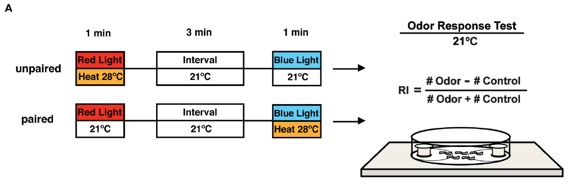
Artificial memory formation with blue light and heat.
Transgenic larvae carrying both Or-ChR2 and Tdc2-dTrpA1 were conditioned with heat (28˚C) and blue light in either unpaired or paired way. The larval olfactory response was then tested at 21˚C on an olfactory test plate.