modeling schizophrenia in flies
Fly model
Disrupted-In-Schizophrenia-1 (DISC1) is a promising genetic factor for a wide range of mental illnesses, including schizophrenia, bipolar disorder, major depression, and autism spectrum conditions (Chubb et al., 2008; Kilpinen et al., 2008; Jaaro-Peled et al., 2009). DISC1 was originally identified as the gene located on chromosome 1 and interrupted by a balanced translocation t(1q42.1;11q14.3) that is linked to psychopathology including schizophrenia, depression, and bipolar disorder in a Scottish pedigree (St Clair et al., 1990; Millar et al., 2000). Cellular and molecular studies have suggested that the DISC1 protein has multiple functions and is located in several distinct domains in neurons, including the centrosome, the postsynaptic density, the mitochondria, and the nucleus (Millar et al., 2005; Kirkpatrick et al., 2006).
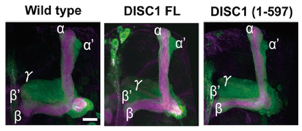
To examine the cellular and molecular functions of DISC1 in living organisms, we have employed a direct expression approach, in which the human DISC1 protein is expressed in the mushroom bodies using an inducible gene expression system (Sawamura et al., 2008). Neither the full-length nor the truncated form of DISC1 causes gross anatomical alterations of mushroom bodies, leaving the characteristic expression of molecular markers including Fas II, a cell adhesion molecule of IgG superfamily, unaltered.
Intriguingly, transgenic flies expressing the full-length DISC1 display disturbance in sleep homeostasis. Wild-type flies exhibit a typical circadian activity with more frequent and extended rest at night than during daytime. Targeted expression of the full-length DISC1 to mushroom body neurons fails to affect sleep in female flies but stimulates the amount of sleep in male flies, while the c-terminally truncated DISC1 does not alter sleep in both male and female flies although a slight increase of the daytime sleep is noticeable in males.
Sleep homeostasis in flies is associated with CREB signaling and CRE-mediated gene transcription (Hendricks et al., 2001; Zimmerman et al., 2008). Intriguingly, studies with tissue culture cells show that DISC1 directly interacts with ATF4/CREB2 transcription factors and a corepressor N-CoR (Sawamura et al., 2008). Furthermore, we have found a direct physical interaction between the human DISC1 and the Drosophila Cryptochepal protein, the fly orthologue of the human ATF4/CREB2, in cultured cells (Sawamura et al., 2008). These results suggest conserved molecular networks of DISC1 interacting partners in the fly and demonstrate the potential of the Drosophila model system for the study of the underlying molecular genetic mechanisms responsible for the major psychotic disorders such as schizophrenia.
A
B
C
A
B
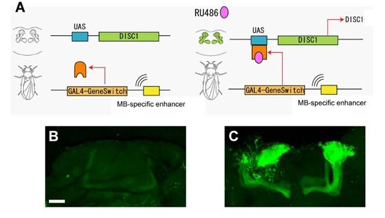
Cell-type and stage specific expression of DISC1 in flies.
(A) Inducible expression of DISC1 in Drosophila mushroom body with MB-GeneSwitch GAL4 construct.
(B, C) Induction of mushroom body expression by the addition of RU486. Green, mCD8::GFP.
Furukubo-Tokunaga, 2009.
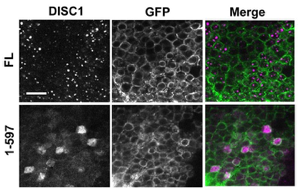
DISC1 expression in Drosophila mushroom body neurons.
(A) Gross morphology of mushroom bodies is unaltered by the expression of DISC1. DISC1 expression was driven by OK107, a mushroom body GAL4 driver. Lobes were visualized with UAS-mCD8::GFP (green) and anti-FASII staining (magenta). Scale bar, 10 μm.
(B) Localization of DISC1 in Drosophila mushroom body neurons. Note that the full-length DISC1 (FL) exhibits punctate nuclear localization, whereas the c-terminal-truncated DISC1 (1-597) localizes diffusely both in the nuclei and cytoplasm. Scale bar, 10 μm.
Sawamura et al., 2008
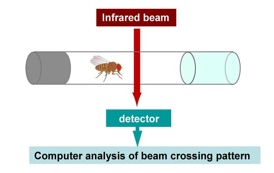
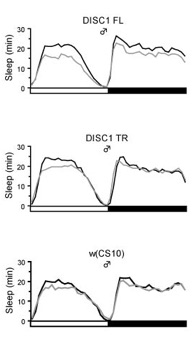
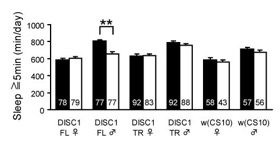
DISC1 stimulates sleep in male flies.
(A) Schematic illustration of Drosophila activity monitoring system.
The circadian activity of flies can be measured by a computerized activity monitor system that counts the numbers of infrared beam crossings by an individual fly placed in a small glass tube.
(B) Daily activity patterns of flies expressing DISC1 full-length (FL) and DISC1 truncated (TR,(1-597) under control of the MB-GeneSwitch. Sleep patterns in flies with (black lines) or without DISC1 induction (gray lines). Sleep was defined as a continuous rest state longer than 5 min.
(C) Total amount of sleep per day. Expression of DISC1-FL, but not DISC1-TR (1-597) leads to longer sleep in males. Black bar, +RU486. White bar, -RU486. Total number of flies used in each group is shown in the bar.
Sawamura et al., 2008; Furukubo-Tokunaga, 2009.
DISC1 in flies
Schizophrenia is a debilitating mental illness affecting 1% of the population worldwide. Increasing evidence suggests that schizophrenia is a subtle disorder of brain development and plasticity. Although its molecular etiology remains still enigmatic, recent advances in human psychiatric genetics have begun to identify genes of candidate genetic risk factors involved in schizophrenia. Modeling the disease in genetically tractable animals is a challenging but increasingly important task.
Drosophila melanogaster has been used successfully as a genetic tool to study the cellular and molecular mechanisms of human neurodegenerative disorders (Lessing and Bonini, 2009). In the application for complex diseases such as mental disorders, it is true that there are limitations in fly models, as in other animal models, particularly with regard to the processes that are only manifested in humans. Thus, Flies and even rodent models would not be ideal to study delusions, hallucinations, thought distortions and other complex mental processes that are manifested explicitly in psychiatric patients. Nevertheless, the molecular and genetical mechanisms that are involved in the generation of the underlying biological alterations, the endophenotypes, of the disease could be studies in non-human models (Gottesman and Gould, 2003; Zoghbi and Warren, 2010; Nestler and Hyman, 2010). Importantly, many of such biological processes are evolutionarily conserved in both vertebrates and invertebrates, and have pivotal roles in regulating the endogenous phenotypes, including memory, attention, sleep, and arousal as well as intracellular signaling cascades such as the cAMP signaling pathway. Information on the molecular and genetical interactions between conserved components of the biological mechanisms in flies could then help to identify novel human genes and molecular pathways that underlie the cognitive and mental deficits in patients.
FURUKUBO-TOKUNAGA LABORATORY
INSTITUTE OF BIOLOGICAL SCIENCES, UNIVERSITY OF TSUKUBA, JAPAN
Update June 2018
dissecting genetic interactions between Disc1 and psychiatric risk factor genes
disc1 suppresses associate memory in flies
Among cognitive deficits found in psychiatric patients, memory deficit is an important endophenotype and has been shown to correlate with genetical susceptibility associated with human DISC1 haplotypes. We expressed DISC1 in larval MB neurons using the GAL4-UAS system and found that overexpression of DISC1 in MB neurons suppressed olfactory associative memory (Furukubo-Tokunaga et al., 2016). We then analyzed memory performance in transgenic larvae expressing DISC1 deletion constructs and revealed that DISC1 amino-terminal domain, which contains several bind sites for PDE4, is critical for memory suppression (Furukubo-Tokunaga et al., 2016). Mosaic analyses with single-cell clones further revealed that overexpression of DISC1 suppresses axonal and dendritic branching of MB neurons (Furukubo-Tokunaga et al., 2016).
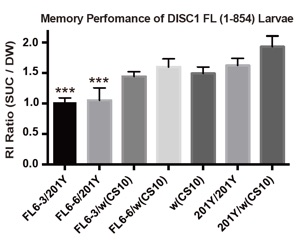
Olfactory memory performance of DISC1 and control larvae.
Memory performance was measured as increment in the olfactory response to the test odor. RI ratio (SUC/DW) = 1.0 corresponds to no learning. DISC1 was expressed in the larval MB neurons by a GAL4 driver, 201Y. Associative memory was suppressed in FL-6-3/201Y and FL-6-6/201Y, two independent DISC1-expressing stocks. ***p < 0.001 with one-way ANOVA and Dunnett’s post hoc test, n = 14 -25.
Furukubo-Tokunaga et al., 2016.
To further investigate the neurodevelopmental functions of DISC1, we examined synapse development using the fruit fly larval NMJs, which exhibit several critical features in common with the excitatory synapses in the vertebrate brain. In particular, the fly NMJs utilize glutamate as the major neurotransmitter and have been used as a powerful model to study the molecular mechanisms of synaptic development and functions.
We found that overexpression of DISC1 with a ubiquitous driver caused moderate shrinkage of the synaptic termini of the motoneurons with a significant reduction in total bouton area. Based on this finding, we conducted screening for psychiatric risk factor genes that might cooperatively function with DISC1 in synaptic development. Through this screening, we found several interacting genes including dysb, the fruit fly homolog of the human Dysbindin (DTNBP1) gene, and dnrx1, the Drosophila homolog of the human Neurexin (NRXN1) gene (Furukubo-Tokunaga., 2016; Pandey et al., 2017).
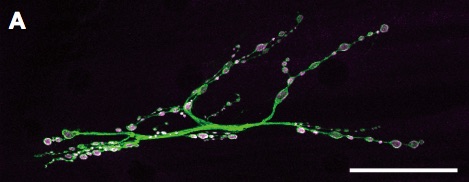
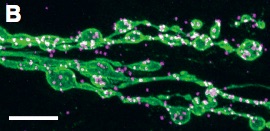
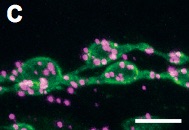
DISC1 interacts with dysbindin in glutamatergic synaptogenesis.
A. Fruit fly larval neuromuscular junction. The fruit fly neuromuscular junctions exhibit several critical features in common with the excitatory synapses in the vertebrate brain. They utilize glutamate as the major neurotransmitter and hence have been used as an excellent model to study the molecular mechanisms of synaptic development and functions. Green, motor neuron termini labeled with an anti-neuronal antibody. Magenta, synaptic boutons labeled with anti-Synaptotagmin. Scale bar, 40 µm.
B, C. Proximity Ligation Assay in fly neuromuscular junctions.
To determine whether DISC1 directly interacts with Dysbindin in synaptogenesis, we applied the Proximity Ligation Assay technique. In this assay, the target proteins are localized with specific primary antibodies, which are then detected with secondary antibodies conjugated to oligonucleotide probes. The attached probes can be bridged through hybridization of connector oligonucleotides only when the two proteins are in close proximity to make direct contact. The annealed oligonucleotides are then closed by ligation into circular DNA molecules, which serve as templates for subsequent rolling circle amplification to be visualized by in situ hybridization. Green, motor neuron termini labeled with anti-HRP. Magenta, Proximity Ligation Assay signals between the Drosophila Dysbindin and the human DISC1 protein. Scale bars, 10 µm (B) and 5 µm (C).
Furukubo-Tokunaga et al., 2016
